Heat of Vaporization of Water Kj Mol
KJK 1 mol 1A. The standard enthalpy of vaporization of water at 100C is 40656 kJ mol1.

Chemistry 201 Using Enthalpy Of Vaporization In Dimensional Analysis Youtube
When 1 mol of water at 100C and 1 atm pressure is converted to 1 mol of water vapor at 100C 407 kJ of heat are absorbed from the surroundings.

. How much heat is absorbed when 184 g of water boils at atmospheric pressure. The molar enthalpy of vaporization for water is 4079 kJmol. The energy absorbed in this process is called heat of vaporization.
The entropy change during vaporization is. What is the normal boiling point of this liquid. The molar heat of vaporization of water is 406 kjmol chemistry A sample of 100 mol H2O g is condensed isothermally and reversibly to liquid water at 100C.
The vaporization is the opposite process of condensation. T oC DvapH kJmol 0 45054 25 4399 40 4335 60 42482 80 41585 100 40657 120. Ask a New Question.
See Example 3 below. Explanation Create a free account to see explanations Continue with Google Continue with Facebook Sign up with email Already have an account. 1 q is the total amount of heat involved 2.
The heat of vaporization of water is 407 kJmol. What is water vaporization. The amount of heat released when 1 mol of vapor condenses is called its molar heat of condensation DHcond.
The molar heat of vaporization equation looks like this. The heat of vaporization of water is 4066 kJmol. The molar heat of vaporization of water is 406 kjmol chemistry A sample of 100 mol H2O g is condensed isothermally and reversibly to liquid water at 100C.
The heat of vaporization of water is 4066 kJmol. See the answer See the answer done loading. View more similar questions or ask a new question.
Then we will convert 1000 C T to Kelvin using the following expression. ΔHvap - ΔHcond H2O g -- H2O l ΔHcond - 407 kJmol Ex prob. H2O1 H0g Compound AH kJmol H0I -286.
Therefore the other deltaHvap value of 440 kJ mol-1 refers to the standard enthalpy of vaporization of water at its standard room temperature 25 degrees Celsius. Find w q U and H for this process. How much energy is absorbed when 303 g of liquid water boils.
Molar mass of water 18 gmole Now we have to calculate the amount of heat released. 19The heat of vaporization for 10 mole of water at 100C and 10 atm is 4056 kJmol. Calculate S for the process H2Ol H2Og at 100C.
When a material in liquid state is given energy it changes its phase from liquid to vapor. Enthalpy values correspond to a nominal pressure of 1 atmosphere. The molar mass of water is 1802 gmol.
The molar heat of vaporization for water is 407 kJmol. 1mathrmmol of water weighs 0018mathrmkg. On the other hand the molecules in liquid water are held together by relatively strong hydrogen bonds and its enthalpy of vaporization 4065 kJmol is more than five times the energy required to heat the same quantity of water from 0 C to 100 C c p 753 J K 1 mol.
First we have to calculate the number of moles of water. Find w q U and H for this process. The room temperature deltaHvap value is.
I believe that the deltaHvap value of 407 kJ mol-1 refers to the standard enthalpy of vaporization of water at its normal boiling point 100 degrees Celsius. The standard enthalpy of vaporization of water at 100C is 40656 kJ mol1. How much heat is absorbed when 213 g of water boils at atmospheric pressure.
At 273 K it has a vapor pressure of 102 mmHg. How much heat is absorbed when 213 g of water boils at atmospheric pressure. Solution for Calculate the enthalpy of vaporization for the phase change from liquid water to water vapor.
The molar heat of vaporization of water is 407 kJ mol. So H2O l -- H2O g ΔHvap 407 kJmol Condensation Condensation is the exact opposite of vaporization. N number of moles of water 0278 mole.
Molar heat values can be looked up in reference books. KJ Calculate the heat energy released when 123 g of liquid mercury at 2500 C is converted to solid mercury at its melting point KJ 4p Constants for mercury at 1 atm heat capacity of Hg 1 280Jmol K melting point. On the other hand the molecules in liquid water are held together by relatively strong hydrogen bonds and its enthalpy of vaporization 4065 kJmol is more than five times the energy required to heat the same quantity of water from 0 C to 100 C cp 753 JKmol.
To get the heat of vaporization you simply divide the molar heat by 18015 gmol. Express this enthalpy of vaporization in joules per gram. The heat of vaporization of water is about 2260 kJkg which is equal to 408 kJmol.
The heat of vaporization of water at 373 K is 407 kJmol. In respect to this what is the enthalpy of vaporization of water. Finally we will calculate the change in the entropy ΔS for this process using the following expression.
Enthalpy of Vaporization of Water The enthalpy or heat of vaporization of water is tabulated as a function of temperature in the following table1 and also represented graphically. The heat of vaporization of water is 4066 kJmol. A liquid has an enthalpy of vaporization of 308 kgmol.
As a gas condenses to a liquid heat is released. 1 What quantity of heat is required to melt 250 g of ice at. Find q w Delta E ΔE and Delta H ΔH for the evaporation of 454 g of water at this temperature at 1 atm.
22 The molar heats of fusion and vaporization for water are 602 kJmol and 406 kJmol respectively and the specific heat capacity of liquid water is 418 JgC. Where heat of vaporization 4066 kJmol q heat released. A 109 JK mol B 109 JK mol C 406 JK mol D 406 JK mol E none of these 20A change of state that occurs in a system is accompanied by 523 kJ of heat which is transferred to the.
The molar heat of vaporization of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid to a gas. Q ΔH vap massmolar mass The meanings are as follows.

How To Calculate Enthalpy For Phase Changes Of Water Mr Pauller Youtube Water Mister Chemistry Notes Calculator
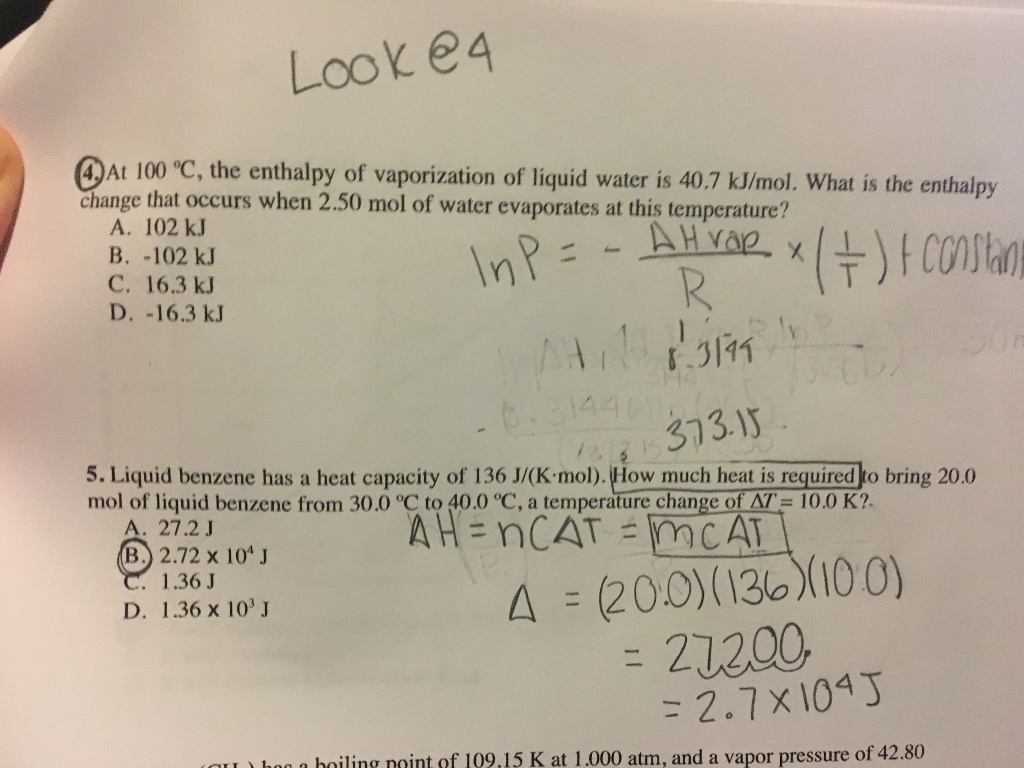
Solved Look E4 Dat 100 C The Enthalpy Of Vaporization Of Chegg Com

Specific Heat J G C 2 06 Ice 4 18 Water 2 03 Steam Molar Heat Of Fusion For Water Kj Mol 6 02 Home Work Help Learn Cbse Forum
No comments for "Heat of Vaporization of Water Kj Mol"
Post a Comment